Abstract
Background: Anti-CD19 Chimeric Antigen Receptor (CAR) T cell therapy has emerged as a mainstay in the treatment of patients with relapsed/refractory (r/r) B-cell acute lymphoblastic leukemia (ALL). While trials have shown encouraging survival rates, up to 50% of patients receiving CART eventually relapse. Identification of risk factors for subsequent relapse is crucial, as this could allow tailored surveillance and treatment approaches for high-risk patients, potentially leading to improved morbidity and mortality. Complete remission with incomplete hematologic recovery (CRi) has been associated with decreased remission duration and overall survival in acute leukemias. As count suppression following CAR T cell therapy is frequently seen, this study evaluated CRi as a prognostic marker for worse relapse-free (RFS) and overall survival (OS) after CAR T cell therapy in comparison to complete remission with hematologic recovery (CR).
Methods: Patients with r/r ALL who achieved a complete morphologic remission after receiving anti-CD19 CAR T cell treatment with the 4-1BB-containing products CTL019 or humanized CART19 in the context of a clinical trial (NCT01626495, NCT02374333, NCT02228096, NCT02435849, NCT02906371) or commercial product (tisagenlecleucel) at Children's Hospital of Philadelphia from 4/2012-4/2019 were identified. Patients who received prior CAR therapy, and those with Trisomy 21 were excluded. Demographic, disease and treatment characteristics, and outcome data were abstracted from the medical record or clinical trial datasets. CR was defined as achieving a morphologic remission with both an absolute neutrophil count (ANC) ≥1,000/µL and a transfusion-independent platelet count ≥100,000/µL at any time between 27-33 days after CAR T cell infusion, whereas those achieving a morphologic remission without complete hematologic recovery were defined as CRi. RFS and OS were described for each cohort. Exposure-outcome association was assessed via the log-rank test and multivariable Cox proportional hazard regression.
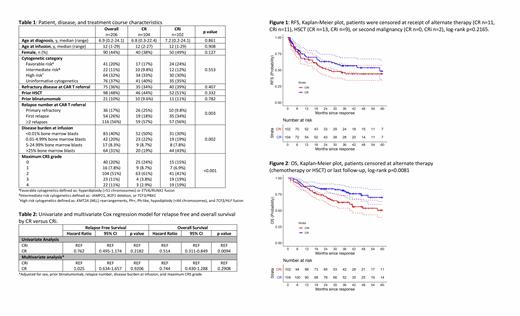
Results: Of the 206 patients included in the analysis, 104 (51%) achieved CR, 102 (49%) CRi. Forty patients (39%) met criteria for CRi with both ANC <1000/µL and platelets <100,000/µL, 40 (39%) for isolated thrombocytopenia, and 20 (20%) for neutropenia alone. Median age at leukemia diagnosis was 7 years (range 0.2-24), and at CAR T cell infusion was 12 years (range 1-29), with 48% undergoing a prior allogeneic stem cell transplant (HSCT), 32% with high risk cytogenetics, and 10% with prior blinatumomab treatment, none of which differed between the CR and CRi groups (Table 1). The number of relapses prior to CAR T cell referral was different between the groups (p=0.003), with more CR patients having been referred for primary refractory disease (25% vs 9.8%), and more CRi patients for first relapse (34% vs 18%). More CRi patients had >25% bone marrow blasts (43% vs 19%) at infusion, whereas more CR patients were MRD-negative (50% vs 30%) at infusion (p=0.002). CRi patients were also more likely to have Grade 3/4 CRS (38% vs 6.7%). Median length of follow-up for patients with CR was 39 months (range 7-89), which was not statistically significantly different than for those patients with CRi (41 months, 11-98, p=0.875). There was no difference in RFS when stratified by hematologic recovery (Figure 1, p=0.2165), with RFS at 36 months for CR of 57% (47-69) and CRi of 46% (36-59). OS was significantly lower (Figure 2, p=0.0081) for those with CRi, with 36-month OS for CR of 81% (74-89), and for CRi of 63% (54-73). In multivariable analysis adjusting for sex, prior blinatumomab, relapse number, disease burden at infusion, and maximum CRS grade, CR was not associated with either RFS (HR 0.76 [95%CI 0.50-1.17] p=0.2182) or OS (HR 0.74 [95%CI 0.43-1.29] p=0.2908), in comparison to CRi, Table 2.
Discussion: Complete remission with incomplete hematologic recovery, manifesting as neutropenia and/or thrombocytopenia, at the first disease assessment following CAR T cell infusion should not be regarded as a harbinger of relapse and demonstrates that patients with CRi have similar probability of durable remission without further therapy. Anticipatory guidance should be provided to patients, and their families, that CRi is more frequently seen in patients who experience high grade CRS, who have high disease burden at infusion, and who are treated in first relapse.
Callahan: Novartis: Speakers Bureau. Rheingold: Optinose: Other: Spouse's current employment; Pfizer: Research Funding. Grupp: Novartis, Roche, GSK, Humanigen, CBMG, Eureka, and Janssen/JnJ: Consultancy; Novartis, Adaptimmune, TCR2, Cellectis, Juno, Vertex, Allogene and Cabaletta: Other: Study steering committees or scientific advisory boards; Novartis, Kite, Vertex, and Servier: Research Funding; Jazz Pharmaceuticals: Consultancy, Other: Steering committee, Research Funding. Maude: Wugen: Consultancy; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal